Our goal was to develop a centralized, cloud-based platform that empowers Regulatory Affairs (RA) teams in the MedTech industry to digitize documentation, automate workflows, and reduce time-to-market by streamlining compliance operations globally.
Strategic Vision: Digitizing Regulatory Complexity for MedTech Excellence
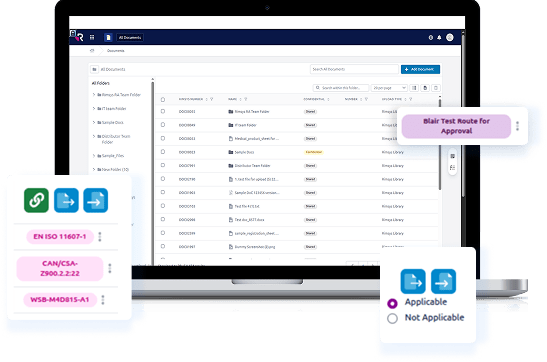
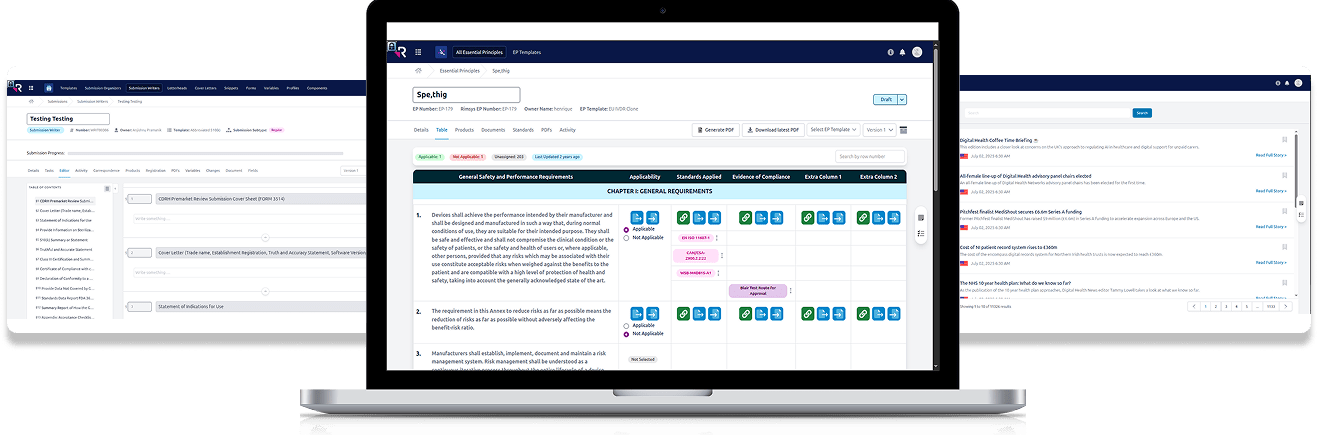
- Centralized Information Architecture that serves as the “single source of truth” for submission data.
- Workflow Automation & Efficiency by logic-driven workflows.
- Localization & Compliance Readiness with the integration of country-specific regulatory entry requirements.
Guided by its vision to simplify complex enterprise workflows through intelligent digital solutions, Codelogicx partnered with Rimsys to reimagine regulatory affairs for the MedTech industry.
The approach focused on building a unified, cloud-based platform that digitizes documentation, centralizes compliance data, and automates key regulatory operations.
By aligning with AWS best practices, implementing agile development, and prioritizing user-centric design, Codelogicx delivered a scalable, secure solution that empowers RA teams to reduce administrative overhead, ensure global compliance, and bring products to market faster.